Ramishetti S, Kedmi R, Goldsmith M, Leonard F, Sprague AG, Godin B, Gozin M, Cullis PR, Dykxhoorn DM, Peer D.
ACS Nano (2015) 9(7):6706-6716.
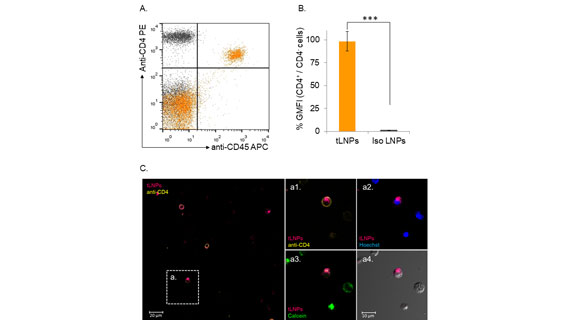
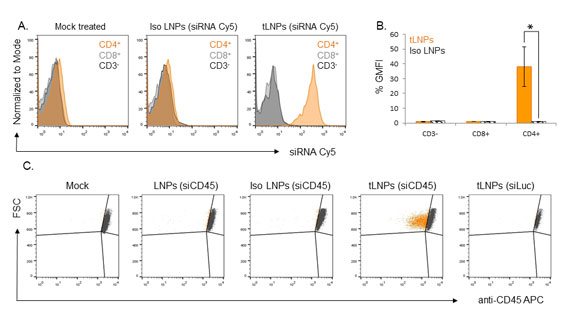
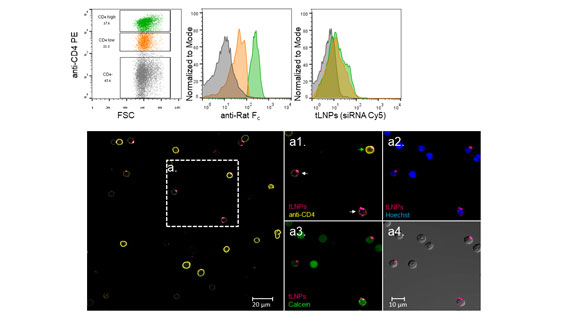
Modulating T cell function by down-regulating specific genes using RNA interference (RNAi) holds tremendous potential in advancing targeted therapies in many immune-related disorders including cancer, inflammation, autoimmunity, and viral infections. Hematopoietic cells, in general, and primary T lymphocytes, in particular, are notoriously hard to transfect with small interfering RNAs (siRNAs). Herein, we describe a novel strategy to specifically deliver siRNAs to murine CD4þ T cells using targeted lipid nanoparticles (tLNPs). To increase the efficacy of siRNA delivery, these tLNPs have been formulated with several lipids designed to improve the stability and efficacy of siRNA delivery. The tLNPs were surface functionalized with anti-CD4 monoclonal antibody to permit delivery of the siRNAs specifically to CD4 T lymphocytes. Ex vivo, tLNPs demonstrated specificity by targeting only primary CD4þ T lymphocytes and no other cell types. Systemic intravenous administration of these particles led to efficient binding and uptake into CD4þ T lymphocytes in several anatomical sites including the spleen, inguinal lymph nodes, blood, and the bone marrow. Silencing by tLNPs occurs in a subset of circulating and resting CD4þ T lymphocytes. Interestingly, we show that tLNP internalization and not endosome escape is a fundamental event that takes place as early as 1 h after systemic administration and determines tLNPs’ efficacy. Taken together, these results suggest that tLNPs may open new avenues for the manipulation of T cell functionality and may help to establish RNAi as a therapeutic modality in leukocyte-associated diseases.